Research
We would like to introduce the contents of our research.
Continuous production technology for biodiesel
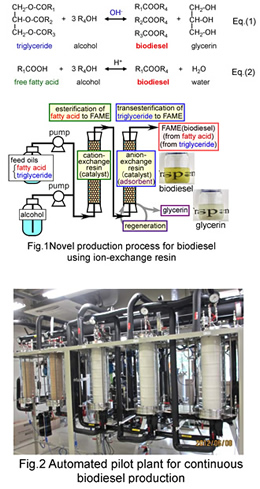
Biodiesel (fatty acid ester) has received much attention as a non-toxic, low emission and renewable energy alternative to petroleum fuels. It is commonly produced by the transesterification of triglyceride, Eq(1), the main constituent of vegetable oils, with alcohol. In the industrial production, homogeneous alkali is widely used as the catalyst for the transesterification. The crude oils usually contain free fatty acid (FFA), formed by triglyceride hydrolysis and FFA reacts with the alkali catalyst to form alkali soap. The soap contaminates the produced biodiesel and lowers the product yield by interfering with the phase separation of biodiesel and by-product glycerin. In most cases, the refined edible oils with less than 1 wt% FFA are used as feedstocks to prevent soap formation, which causes an increase in the production cost. On the other hands, trace amounts of glycerin remaining in the biodiesel after the phase separation gives rise to serious problems and damages for diesel engines. The downstream purification to remove the impurities is necessary to meet the strict international standard specifications and causes a further increase in the production cost.
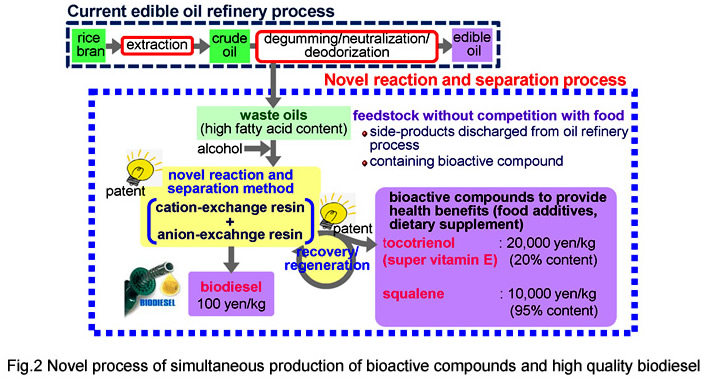
In this research, we propose a new technology to produce high-quality biodiesel from oils with high FFA content, 1-100 wt%, which have never been used as feed stock in the current technology. In the process (Fig.1), an expanded-bed reactor packed with a cation-exchange resin catalyst for esterification of FFA, Eq.(2) and that packed with the anion-exchange resin catalyst for transesterification of triglyceride were connected in series. Both FFA and triglyceride in the oil were completely converted to biodiesel without soap formation. In addition, all by-products, glycerin and water, were adsorbed on the anion-exchange resin and the effluent from the process was free from them. The effluent fully met the international standard specifications without any downstream purification processes except for removing methanol. The glycerin adsorbed on the resin was completely recovered as a transparent methanol solution during regeneration of the resin. We have already completed pilot-scale plant for continuous production (Fig.2).
Learn more about Continuous production technology for biodiesel
High-efficiency recovery technology for vitamin E
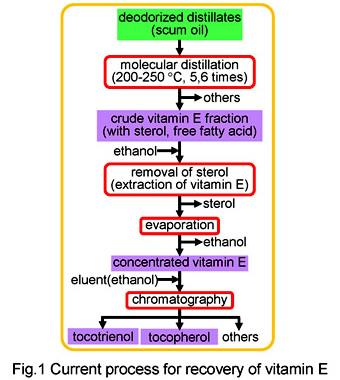
Vitamin E, tocotrienol and tocopherol, has received much attention as bioactive compound to provide a health benefit, such as antioxidant activities, cholesterol lowering, anti-cancer effects and protection against atherosclerosis. Especially, tocotrienol having much higher activity than tocopherol is containing at low concentration in oils extracted from a few plants such as palm and rice bran. At present, tests are being conducted using a technique for separating and recovering vitamin E from these oils using multi-stage molecular distillation at 100?250°C, and chromatographic separation. However, substances in the vitamin E group have low thermal stability, and are susceptible to thermal denaturation. As a result, low recovery rates are a problem with the above method.
In this research, we propose an adsorption/desorption method using ion-exchange resin, as a new recovery technique which does not involve a molecular distillation step. With this technology, it is possible to selectively recover vitamin E under mild conditions of ordinary atmospheric pressure and temperature, and then achieve recovery at high concentration by supplying supernatant. We have already achieved recovery rates of 80% or more, which are two or more times higher than the conventional method. At present, we are working to develop a simultaneous manufacturing process for biodiesel fuel and vitamin E using oily biomass as a raw material.
Research on Control Methods for Oxidation and Antioxidant of lipid, Edible Oils and Other Lipids
It is said that greenhouse gas emissions from food loss are as high as those from automobiles. Many lipid-containing foods have expiration dates based on flavor deterioration caused by oxidation, and if this oxidation can be effectively controlled, the expiration date can be extended.
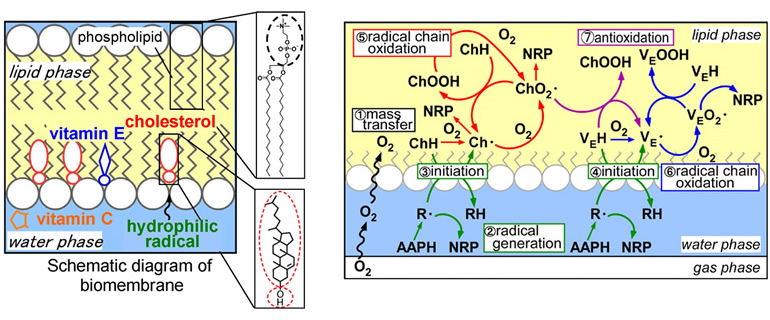
On the other hand, the mechanism of lipid oxidation in food is a complex interplay of radical species derived from the constituent substances. The reactions between lipids and water- and lipid-soluble antioxidants proceed in a complex chain reaction network, producing oxidation and antioxidant reactions as well as oxidation-promoting reactions. Therefore, it is very difficult to predict decomposition and take preventive measures. Therefore, for effective oxidation prevention, it is important to analyze such complex mechanisms.
In this research, we are focusing on the behavior of the major reactive oxygen species (radicals) that govern lipid oxidation, and are working to predict their lifespan by making full use of mathematical model analysis combined with kinetic or statistical processing. In order to control the complex behavior of radicals, we have introduced an engineering approach to develop antioxidant methods that can efficiently inhibit the progression of oxidation.
In addition, oxidation of biomembrane lipids, which constitute human tissue cells, is known to contribute to lifestyle-related diseases such as cancer and atherosclerosis, and this oxidation proceeds based on a radical chain mechanism similar to the oxidation of edible oils. Therefore, we are conducting research with a view to applying this technology to disease prevention based on oxidation in vivo in the future.
Research on selective oxidation control methods for the chemical industry
Oxidation is the most important reaction, accounting for 30% of all chemical processes. In addition, recent examples of biomass utilization have shown that high added value is possible through oxidation from raw materials that would otherwise be discarded. However, in the current situation, oxidants with high environmental impact are often used to produce only the desired product, and the oxidation reaction process is said to be one of the most environmentally polluting processes. The ideal method to overcome this problem is to use only oxygen (air) as an oxidant to produce only the desired chemical product. On the other hand, oxygen, as it is, attacks various places of the raw material molecules that are not the target of the process, and often produces products other than the target ones, or decomposes them into carbon dioxide at the end. Therefore, it is important to establish a method to convert this oxygen into an active species that produces only the desired chemical product under conditions of low environmental impact.
In this study, we focus on green oxidation reactions with oxygen and water in order to proceed with selective oxidation reactions under mild conditions. The combination of oxygen and water is clean and also has a very low environmental impact. Therefore, we are attempting to design a catalytic reaction that produces active species capable of selective oxidation in order to use them to proceed only with the desired oxidation. We are also investigating the control of reactive oxygen species produced in water, focusing on plasma irradiation as a means of obtaining the desired reactive species without a catalyst.
Development of advanced resource conversion process for renewable raw materials using solid catalysts and its kinetic model analysis technology
Bioethanol and green methanol are currently attracting attention, and furthermore, longer carbon chain hydrocarbons and alcohols are expected to be in demand for fuel and chemical product applications in the future. Among these, isobutanol has recently attracted attention for its properties as a drop-in fuel. Therefore, the production of these aliphatic alcohols from renewable resources will lead to the realization of biorefineries.
We propose a method to upgrade bioethanol as base feedstock and green methanol as feedstock for carbon chain elongation to desired carbon chain alcohols, especially C4 alcohols, by gas phase catalytic flow reaction system.
We are also working on upcycling technology for waste plastics, and are engaged in catalytic reaction analysis and process design with the aim of converting plastics into advanced resources into lubricants and liquid fuels by breaking them down at the desired location.
To achieve this, we are working to visualize bottlenecks in the reaction process by using a top-down reaction kinetics model analysis. This mathematical modeling allows us to focus on the dominant processes from the entire process to the nano-order surface reactions, which enables us to elucidate the mechanisms even in areas that cannot be visualized. Based on the information obtained from this analysis, we design catalytic processes for the desired reaction to proceed.



